Draper to Develop an Advanced, Multi-Organ, Microphysiological System Architecture
CAMBRIDGE, Mass.— The field of microphysiological systems has continued to evolve during the past 20 years, doing so in response to the tremendous challenges facing pharmaceutical development and national biodefense initiatives. This includes the high cost, lack of availability and limited human clinical relevance of animal models.
Draper is responding to these national security challenges and announces the $34.1 million award of the Evaluation of Treatments using High-throughput Multi-Organ Systems (ETHOS) program.
The Defense Threat Reduction Agency (DTRA), Joint Science and Technology Office (JSTO), Vaccines/Therapeutics Division (CBM) is the government sponsor for this program.
DTRA’s Research and Development Directorate provides science, technology and capability development investments that maintain the U.S. military’s technological superiority in countering weapons of mass destruction and emerging threats, mitigate the risks of technical surprise and respond to the warfighters’ urgent technical requirements. (Sources: https://www.dtra.mil/About/Mission/Research-and-Development/, https://www.dtra.mil/Portals/125/Documents/CB/DTRA-JSTO_E-book_PDF.pdf)
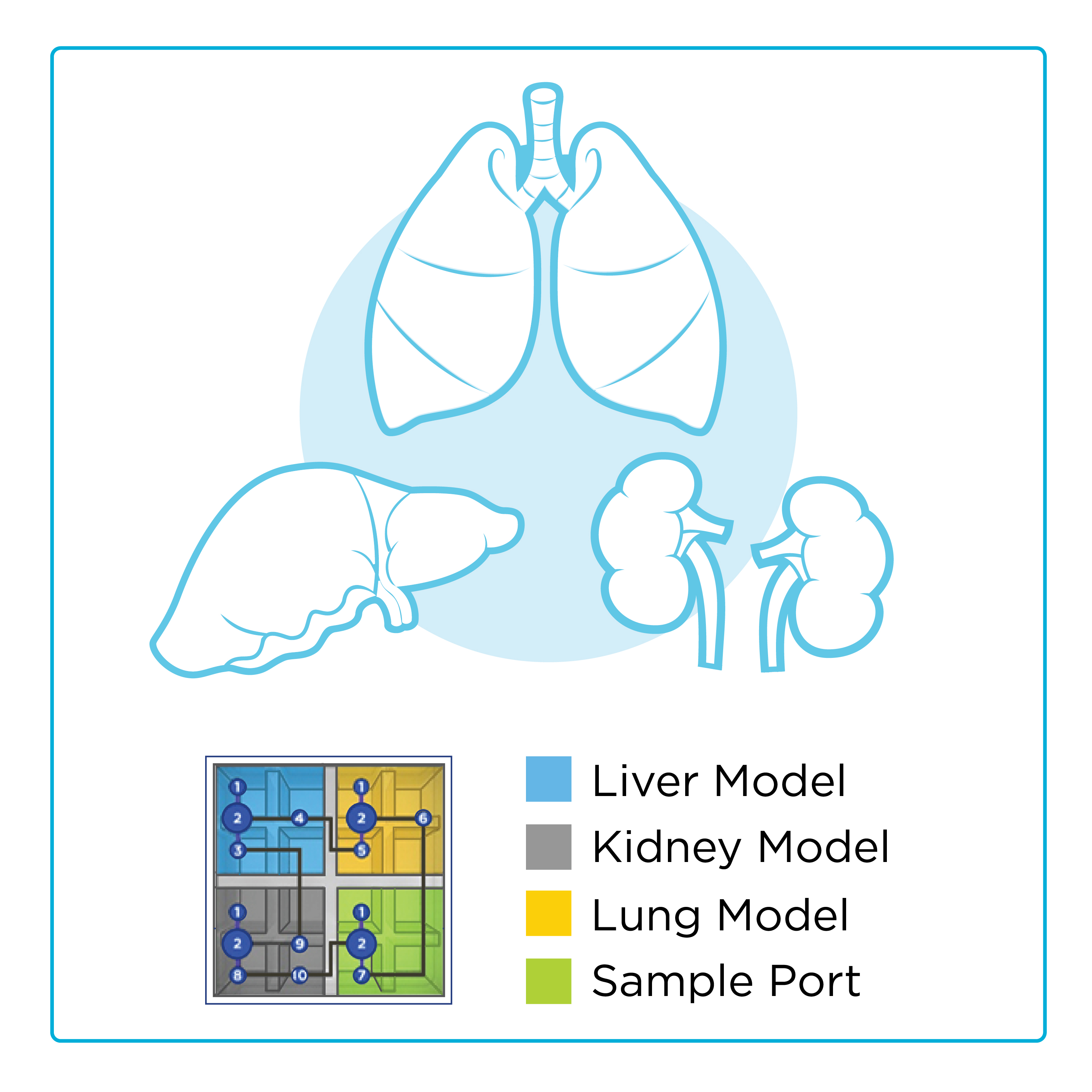
The overall objectives of the ETHOS program are to create a scalable microphysiological system architecture that consists of interacting immune-competent micro-organ (lung, liver, kidney) models to predict the body’s disease response and test medical countermeasure (MCM) responses.
Draper’s advanced models will include parenchymal, vascular and innate immune components to capture a broad range of biological responses. The technical approach will be to create a new multi-organ platform to understand disease progression of high-priority BSL-3 agents and determine the safety and efficacy of MCM treatments.
“Draper looks forward to advancing these MPS-based capabilities for DTRA and their U.S. government partners in order to protect the warfighter against high-priority threats and accelerate our response to future threats,” said Roger Odegard, the Draper ETHOS program manager.
DTRA has recognized that significant research and development in the MPS technology area will improve the state-of-the-art capability to analyze the pathogenesis of disease and injuries that result from exposure to high-priority pathogens and toxins.
Additionally, next-generation MPS technologies and systems will be critical in the evaluation of efficacious and safe medical countermeasures (MCM) to combat these threats and allow the military to be equipped to meet its mission. This will include investigating and characterizing the mechanisms of infection/inflammation/tissue damage across relevant population demographics (e.g., military age, sex, co-morbidities and previous exposure) and under varying conditions (e.g., exposure levels, drug doses and time scales) for a variety of threats. These technology innovations represent a state-of-the-art tool for pharmaceutical solutions that has the potential to significantly accelerate the process of evaluating effective and safe MCMs for emerging, naturally or engineered threats.
This project has been funded in whole or in part with federal funds from the Department of Defense (DoD); The Defense Threat Reduction Agency (DTRA); Joint Science and Technology Office (JSTO), Vaccines/Therapeutics Division (CBM) through the Medical CBRN Defense Consortium (MCDC) Agreement No. MCDC2201-003.
Released November 21, 2024