Draper’s Heart Valve for Children Earns Top Award
CAMBRIDGE, MA—A heart valve that can grow with a child has earned Draper top honors as an innovative medical device technology.
In its award announcement, the American Society for Artificial Internal Organs (ASAIO) recognized Draper for its pediatric heart valve and its innovative growth adaptive design.
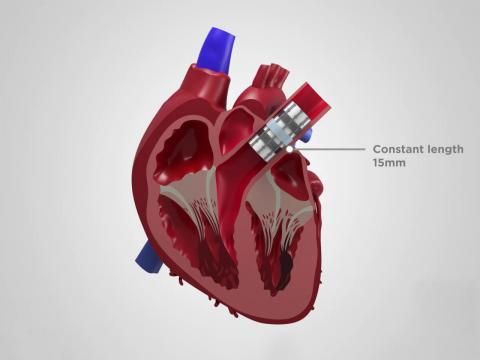
Daniel King, Senior Member of Draper’s Technical Staff, worked on the team that is developing the pediatric heart valve (PHV)—an invention the team calls the LEAP™ Valve, which stands for Low-force Expanding/Adaptable Pediatric Valve.
“Draper is helping children, families and physicians through the development of the passively expanding LEAP Valve which would reduce the number of surgeries needed for 0-to-6-year-old children born with defects,” said King.
A panel of professionals with expertise in regulations, reimbursement, intellectual property and venture capital selected King’s presentation as the best among those presented at the Medical Device Entrepreneur’s Forum at ASAIO’s annual conference.
Children born with congenital heart valve defects number in the thousands each year, yet there are no prosthetic heart valves designed specifically for babies. The LEAP Valve is designed to grow with the child, with at least a two-fold diameter expansion. The initial goal is to serve the most critical unmet need, which are children as young as infants through age 6, who require valve diameters as small as 7 millimeters, half the size of any valve currently on the market.
While an expandable and adaptable prosthetic heart valve fills a significant technology gap for pediatrics, it can also bring other benefits to patients and families. A single surgery to fix a child’s heart valve defect can cost upwards of $200,000. That figure can balloon many times over if a surgeon implants a prosthetic heart valve that isn’t designed to expand in size with the child’s growing heart. Other advantages may include fewer days in the hospital, fewer complications following surgery and lower total costs.
“We envision this technology serving the youngest patients who currently don’t have valve replacement options, while eliminating the need for at least one surgical or invasive procedure to expand or replace the valve,” said King. “Our hope is to improve the long-term prognosis for the child and reduce the overall disease-associated costs to families.”
A multicenter study is underway to evaluate the efficacy and safety of the growth-adaptive pediatric heart valve in a growing animal model. The goal is to develop the heart valve to the investigational device exemption stage, positioning it for first-in-human early feasibility studies. Ultimately, the plan is to apply for FDA approval through the agency’s Humanitarian Device Exemption pathway.
The heart valve is under development in collaboration with pediatric clinicians, including Dr. Sitaram Emani at Boston Children’s Hospital, and Dr. Michael Portman, a cardiologist at Seattle Children’s Hospital. Corin Williams of Draper is the principal investigator of the LEAP heart valve.
This work was supported by the U.S. Army Medical Research Acquisition Activity, through the program “Growth-Adaptive Pediatric Heart Valves: Addressing a Critical Unmet Need for Infants and Young Children That Saves Lives and Reduces Surgeries” under award number W81XWH2010295. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
In conducting research using animals, the investigators adhere to the laws of the United States and regulations of the Department of Agriculture.
The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office.
Released June 28, 2021